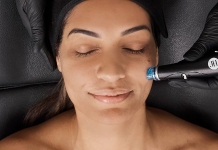
Dermalogica's microneedling system achieves FDA clearance

Dermalogica has secured clearing from the U.S. Food and Drug Administration (FDA) for its PRO Pen Microneedling System as a Class II medical device.
To achieve this clearance, the device was put through scientifically robust clinical studies and had to meet strict FDA requirements.
Commenting, Robert J. Bianchini, Vice-President Technology & Innovation at Dermalogica, says:
"I am excited to have led the team responsible for FDA 510K Clearance of Dermalogica's new Pro Pen Microneedling System. The updated design represents a true milestone in Dermalogica's expansion into Medical Spas and follows closely behind our highly successful Exobooster Lactobacillus Exosome Treatment Serum.”
Aurelian Lis, Dermalogica's Chief Executive Officer, adds:
"Dermalogica has always advanced skin health, but FDA clearance marks our evolution into a new arena as a medical device innovator. By moving beyond traditional skincare and bringing our innovation mindset into medical aesthetics, we're not just celebrating a brand milestone - we're raising the bar for the entire industry with treatments practitioners are proud to deliver and consumers can truly trust.”
Dermalogica

-(1)-13444.jpg)



-13446.jpg)